Low sexual desire is one of the most common yet least openly discussed challenges in female sexual health. Many women experience a persistent decline in sexual desire that affects intimacy, confidence, and overall quality of life, yet struggle to find clear answers or effective treatment options. In many cases, this loss of desire is dismissed as a normal part of aging, stress, or hormonal change, leaving women feeling unheard and untreated.
Female sexual dysfunction is complex and often misunderstood. While hormone therapy and bioidentical hormones can be helpful for some women, they do not address every cause of low libido. For many patients, sexual desire is less about hormone levels or blood flow and more about how the brain and nervous system regulate arousal and motivation.
Here, we’ll explore PT-141, also known as Bremelanotide, an FDA-approved treatment for Hypoactive Sexual Desire Disorder in premenopausal women, and explain how female sexual desire works, why traditional approaches sometimes fall short, and how PT-141 fits into a modern, brain-based approach to treating low libido and sexual distress. You’ll also learn who may benefit from this treatment, important safety considerations, and how Boston Medical Group supports women seeking medically guided solutions for sexual wellness.
Understanding Female Sexual Desire and Sexual Dysfunction
Female sexual desire is not a single switch. Rather, it’s the result of a dynamic interaction between the brain, nervous system, hormone levels, metabolic health, emotional state, and life stage.
When one or more of these systems becomes disrupted, women may experience changes in sexual interest, arousal, or satisfaction.
What Is Hypoactive Sexual Desire Disorder (HSDD)?
Hypoactive Sexual Desire Disorder is defined as a persistent or recurrent absence of sexual desire that causes personal distress and is not better explained by another medical condition, medication, or situational factor. It is one of the most common female sexual dysfunctions and primarily affects premenopausal women, though it can occur at any age.
Clinically, HSDD is evaluated using validated tools such as the Female Sexual Function Index (FSFI) and the Female Sexual Distress Scale (FSDS). These instruments help clinicians assess both the physical aspects of sexual function and the emotional distress associated with low desire.
Importantly, HSDD is not about a woman’s partner, relationship satisfaction, or moral values. It is a recognized medical disorder rooted in neurochemical signaling within the central nervous system, particularly in the hypothalamus and related brain regions that regulate motivation and reward.
Related diagnostic terms may include:
- Female sexual desire disorder
- Female sexual arousal disorder
- Sexual arousal disorder
- Female sexual interest/arousal disorder
While terminology has evolved, the core issue remains the same: diminished sexual desire that negatively impacts quality of life.
Common Causes of Low Sexual Desire in Women
Low libido rarely has a single cause. Instead, it often reflects overlapping biological and neurological factors, including:
- Hormonal changes associated with aging, perimenopause, menopause, or postpartum periods
- Shifts in hormone levels, even when standard lab values appear “normal.”
- Genetic predispositions affecting neurotransmitter or receptor activity
- Metabolic conditions such as diabetes or insulin resistance
- Declines in overall metabolic health
- Chronic stress, fatigue, or nervous system dysregulation
Traditional approaches often emphasize estrogen, testosterone, or other hormonal therapies. While hormone replacement therapy and bioidentical hormones can be helpful for some patients, many women continue to experience low sexual desire despite optimized hormone therapy.
This is because sexual desire originates in the brain, not the bloodstream.
The hypothalamus, an area of the brain rich in melanocortin receptors, plays a central role in sexual motivation. When signaling in this system is impaired, increasing blood flow or adjusting hormone levels alone may not restore desire. This understanding is what led researchers to explore melanocortin receptor agonists like PT-141 as a new approach to female sexual health.
Because PT-141 acts directly on the nervous system, rather than peripheral blood flow, it can address the root neurological drivers of sexual desire and arousal, offering a fundamentally different option for women whose symptoms persist despite conventional treatments.
What Is PT-141 (Bremelanotide)?
PT-141, clinically known as Bremelanotide, is a synthetic peptide developed specifically to address disorders of sexual desire by acting on the brain rather than the vascular system or hormone levels alone. It belongs to a class of compounds known as melanocortin receptor agonists and is one of the few pharmacologic treatments designed to directly influence sexual motivation and arousal at the neurological level.
Unlike many traditional treatments for female sexual dysfunction that focus on hormone replacement therapy or improving blood flow, PT-141 targets signaling pathways within the central nervous system. This makes it especially relevant for women whose low sexual desire persists despite balanced hormone levels or the use of hormonal therapies.
PT-141 is FDA-approved under the brand name Vyleesi for the treatment of Hypoactive Sexual Desire Disorder in premenopausal women. It is administered as an on-demand subcutaneous injection and is intended to be used prior to anticipated sexual activity, rather than as a daily medication.
From Melanotan-II to Bremelanotide
The development of PT-141 traces back to early research on melanotan-II, a synthetic analog of alpha-melanocyte-stimulating hormone. Melanotan-II was initially studied for its effects on pigmentation through melanocortin receptor-1 (MC-1R). During early trials, researchers observed unexpected increases in sexual arousal and spontaneous desire in some subjects.
This discovery led to the refinement of a more targeted compound—PT-141—a melanocortin agonist designed to minimize pigment-related effects while maximizing activity at melanocortin receptors involved in sexual behavior. In particular, Bremelanotide was engineered to act primarily on melanocortin-receptor-4 (MC4R) and melanocortin-receptor-3 (MC3R), which are concentrated in areas of the brain that regulate motivation, reward, and sexual interest.
Following multiple phase II and phase III clinical trials, PT-141 demonstrated statistically and clinically meaningful improvements in sexual desire and reductions in sexual distress among women diagnosed with HSDD. These results ultimately led to FDA approval for female hypoactive sexual desire disorder, marking a significant milestone in female sexual health.
How PT-141 Differs from Traditional Sexual Health Treatments
Most conventional treatments for sexual dysfunction focus on downstream physical responses, such as increasing genital blood flow or adjusting circulating hormone levels. While these approaches can be effective in certain contexts, they do not address the neurological initiation of sexual desire.
PT-141 works differently. As a melanocortin agonist, it activates receptors in the brain that signal sexual interest and arousal before any physical response occurs. This distinction is especially important for women whose primary concern is low libido rather than an inability to become physically aroused.
Because PT-141 does not rely on vascular dilation, its effectiveness is not dependent on blood flow. This makes it a valuable option for patients with metabolic conditions such as diabetes or those who have not responded to hormone therapy alone.

How PT-141 Works in the Female Brain and Nervous System
Sexual desire begins in the brain. Long before physical arousal occurs, the nervous system processes motivation, anticipation, and emotional readiness. PT-141 is designed to influence this process at its source by interacting with the melanocortin system within the central nervous system.
The Role of Melanocortin Receptors in Sexual Desire
Melanocortins are naturally occurring peptides that regulate a wide range of physiological functions, including appetite, energy balance, mood, and sexual behavior. These peptides exert their effects by binding to melanocortin receptors located throughout the brain and nervous system.
PT-141 acts as a melanocortin receptor agonist, primarily active at the MC3R and MC4R receptors in the hypothalamus. The hypothalamus serves as a control center for sexual behavior, integrating hormonal signals, emotional cues, and sensory input to regulate sexual desire and arousal.
When PT-141 activates these receptors, it enhances signaling within neural pathways associated with motivation and reward. This activation increases sexual interest and responsiveness, even in the absence of external stimulation.
A Brain-Based Approach to Sexual Arousal
PT-141 initiates sexual arousal through the nervous system when it stimulates melanocortin receptors in the brain. It promotes the release of neurotransmitters such as dopamine, which play a central role in desire and pleasure. This way, it does not depend on restoring sexual function by just improving blood flow to the genital tissues.
This mechanism helps explain why PT-141 can be effective for women who have normal hormone levels, yet experience diminished libido. It also clarifies why hormone replacement therapy alone may not restore sexual desire in some patients, particularly when the underlying issue involves neural signaling rather than endocrine imbalance.
Peptide therapy with PT-141 does not force a physical response. Instead, it restores the brain’s ability to generate sexual interest naturally, allowing arousal to develop in a way that feels more spontaneous and emotionally connected.
Why Central Nervous System Targeting Matters
Female sexual dysfunction is often multifactorial, involving emotional health, stress response, metabolic health, and neurological signaling. By acting on the arousal pathways through the central nervous system rather than peripheral systems alone, PT-141 addresses a critical but often overlooked component of female sexual health.
This brain-first mechanism is especially relevant for women experiencing sexual desire disorders related to aging, perimenopause, or hormonal decline, where traditional hormone therapy or blood flow–focused treatments may fall short. It also allows PT-141 to complement other therapies rather than replace them, offering flexibility within individualized treatment plans.
In fact, because of its success in enhancing sexual arousal in women, PT-141 has also seen increased off-label usage to support therapies in men’s sexual health, as either treatment for erectile dysfunction or premature ejaculation. However, its use for treating male sexual health is not yet approved, even if it’s been shown in several trials and clinical settings.
Clinical Benefits of PT-141 for Women
PT-141 offers a distinct set of benefits for women experiencing low sexual desire, particularly when traditional approaches such as hormone therapy or lifestyle changes have not been effective. Because it works through the brain and central nervous system, its effects extend beyond physical arousal to address motivation, interest, and emotional engagement.
Increased Sexual Desire and Sexual Arousal
One of the primary benefits of PT-141 is its ability to restore sexual desire at the neurological level.
Women using PT-141 often report:
- Increased spontaneous sexual thoughts
- Greater interest in initiating sexual activity
- Improved responsiveness to sexual stimulation
- A stronger sense of anticipation and motivation
This makes PT-141 particularly useful for women whose symptoms are best described as low desire rather than difficulty with lubrication or physical response.
Improvements Demonstrated in Clinical Trials
PT-141’s approval for Hypoactive Sexual Desire Disorder was based on multiple randomized, placebo-controlled clinical and Phase 3 trials involving premenopausal women. These studies measured outcomes using well-established diagnostic tools for female sexual dysfunction.
In clinical research, women treated with PT-141 showed:
- Statistically significant improvements in sexual desire scores
- Increased number of satisfying sexual events
- Higher scores on the Female Sexual Function Index
- Reduced distress as measured by the Female Sexual Distress Scale
These improvements were not limited to physical measures alone. Many patients reported meaningful changes in how they felt about their sexual relationships and their own sexual identity, underscoring the importance of addressing both desire and distress together.
Emotional, Psychological, and Quality-of-Life Benefits
Low libido or sexual desire often affects more than intimacy, as women report impacts on self-esteem, mood, and emotional connection with a partner. So, when a treatment like PT-141 addresses the brain-based drivers of sexual motivation, it may help break the cycle of frustration and avoidance that can develop over time with female sexual dysfunctions.
Reported benefits may include:
- Reduced anxiety related to sexual activity
- Improved confidence and sense of sexual well-being
- Greater emotional connection during intimacy
- Increased number of satisfying sexual events and improved sexual wellness
These effects highlight why PT-141 is viewed as a treatment for sexual desire disorder rather than simply a tool for physical arousal.
Who Can Use PT-141 — and Who Should Not
PT-141 is not a one-size-fits-all solution. Appropriate patient selection and medical oversight are essential to ensure safety and effectiveness. A thorough evaluation helps determine whether this treatment aligns with a patient’s medical history, symptoms, and overall health.
Ideal Candidates for PT-141
PT-141 may be appropriate for women who meet specific clinical criteria and experience ongoing distress related to low sexual desire. Ideal candidates often include:
- Premenopausal women diagnosed with Hypoactive Sexual Desire Disorder
- Women with persistent low sexual desire not explained by relationship issues or medications
- Patients who have not responded adequately to hormone therapy or hormonal therapies
- Women whose hormone levels are within normal ranges, but symptoms persist
- Patients seeking an on-demand, non-daily treatment option
Because PT-141 acts on the central nervous system, it may be especially helpful for women whose symptoms are linked to neurological signaling, stress response, or changes associated with aging or perimenopause.
Women Who Should Avoid or Use Caution with PT-141
Certain medical conditions may make PT-141 inappropriate or require careful monitoring. Women who should avoid PT-141 or only use it under close medical supervision include those with:
- Uncontrolled hypertension
- Significant cardiovascular disease
- Known hypersensitivity to melanocortin peptides
- A history of severe adverse reactions to injectable therapies
- Pregnancy or breastfeeding
PT-141 can cause temporary increases in blood pressure shortly after administration, which is why cardiovascular screening is an important part of the evaluation process.
The Importance of Medical Evaluation and Ongoing Support
Before starting PT-141, patients should undergo a comprehensive assessment that may include:
- Review of medical history and current medications
- Evaluation of sexual symptoms and issues by using the female sexual distress scale
- Discussion of prior hormone replacement therapy or treatments
- Screening for metabolic conditions such as diabetes
At Boston Medical Group, patients are supported by a dedicated medical support team that provides education, dosing guidance, and follow-up care. This ensures that PT-141 is used safely and effectively as part of a broader approach to female sexual health.
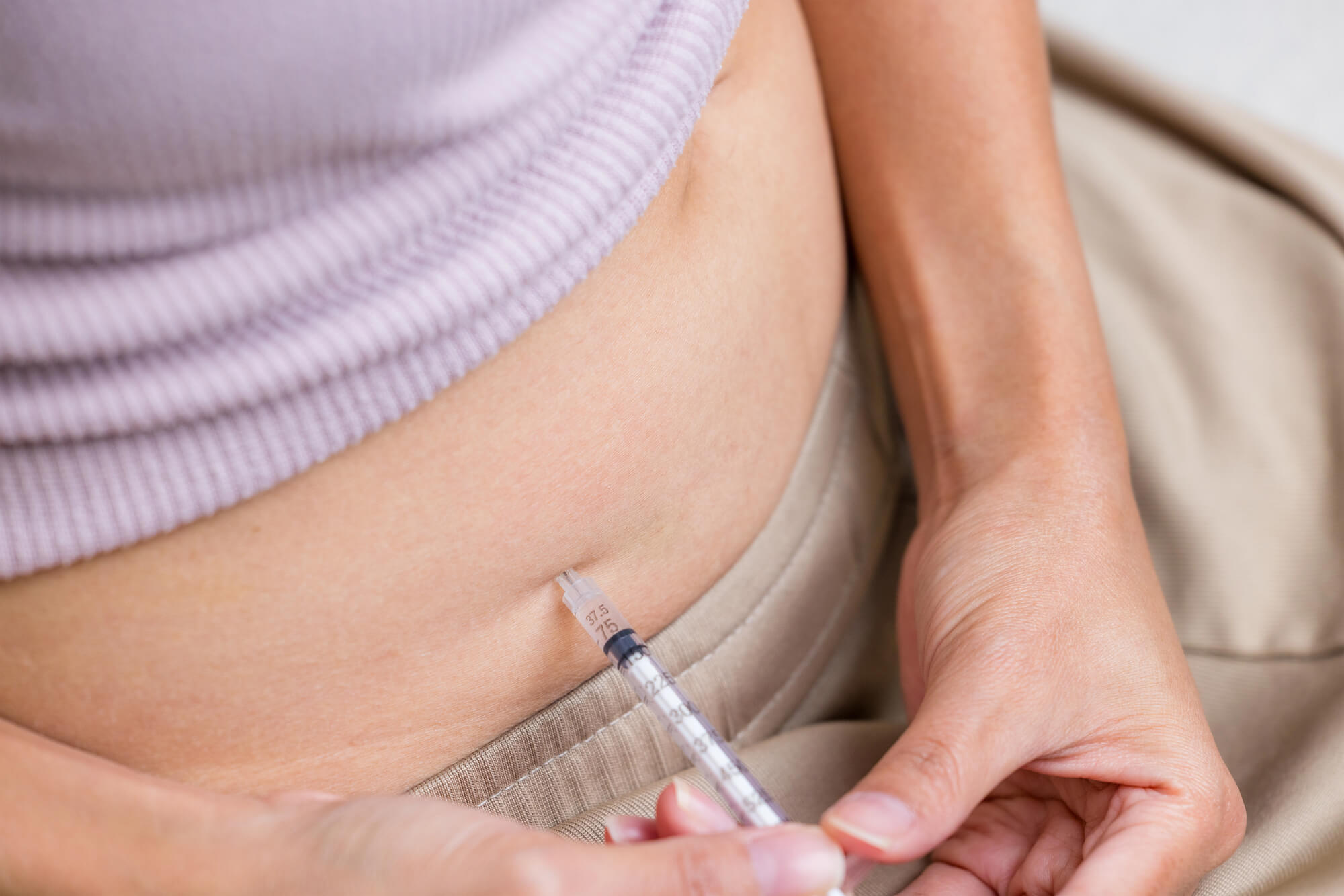
How PT-141 Is Taken (Dosing and Administration)
PT-141 is designed for on-demand use, offering flexibility and control for women seeking treatment for sexual desire disorder. Unlike daily medications or long-term hormone therapy regimens, PT-141 is administered only when needed, prior to anticipated sexual activity.
Subcutaneous Injection and Self-Administration
PT-141 or Bremelanotide (BMT) is administered as a subcutaneous injection, meaning it is injected just beneath the skin rather than into a muscle or a vein. The medication is typically injected into the abdomen or upper thigh using a prefilled autoinjector device.
Key features of PT-141 administration include:
- Designed to self-administer at home
- Delivered via a short, fine needle
- No need for intravenous access or clinical visits
- Minimal preparation required
Most patients find the injection process straightforward after initial instruction. The autoinjector format helps ensure accurate dosing and reduces user error, which improves consistency and confidence.
Timing and Dosing Guidelines
Subcutaneous BMT or PT-141 is intended to be used approximately 45 minutes before sexual activity, allowing time for the medication to engage the central nervous system and activate melanocortin receptors in the brain.
Standard dosing guidelines generally include:
- One dose per 24-hour period
- No more than eight doses per month
- Use only as needed, not on a daily schedule
Food intake does not significantly affect its effectiveness. This allows for greater spontaneity compared to some oral sexual health medications.
Why Injection Is the Preferred Delivery Method
Earlier research explored intranasal dosing for Bremelanotide, but variability in absorption and a higher incidence of adverse events limited its usefulness. Subcutaneous injection provides more reliable bioavailability and predictable improvements in the female sexual function index.
Compared to intranasal doses, subcutaneous BMT injections offer:
- More consistent absorption
- Greater control over dosing
- Reduced variability in blood pressure response
- Improved clinical reliability
For these reasons, subcutaneous administration remains the FDA-approved and clinically preferred method for PT-141 therapy.
Side Effects and Safety Considerations
Like all prescription treatments, PT-141 has potential side effects. Most are mild to moderate and occur shortly after administration. Understanding what to expect helps patients use the medication safely and with confidence.
Common Side Effects
The most frequently reported side effects of PT-141 are related to its action on the central nervous system and melanocortin receptors. Common reactions may include:
- Nausea, especially after the first few doses
- Flushing or warmth in the face or chest
- Headache
- Mild injection site reactions such as redness or tenderness
Nausea is the most commonly reported side effect, but it often decreases with continued use as the body adjusts. Some patients find that resting briefly after the injection or adjusting the timing helps minimize discomfort.
Less Common or More Serious Reactions
Although uncommon, some patients may experience more pronounced adverse events, including:
- Vomiting or dizziness
- Temporary increases in blood pressure
- Elevated heart rate shortly after injection
- Gradual skin darkening with prolonged or frequent use
These effects are typically transient, but they underscore the importance of medical screening and adherence to dosing guidelines.
Safety Screening and Monitoring
Before starting PT-141, patients should undergo a medical evaluation to assess cardiovascular risk, current medications, and overall health status. This is particularly important for women with a history of hypertension, metabolic conditions, or nervous system sensitivity.
At Boston Medical Group, patients have access to a medical support team that provides:
- Clear dosing instructions
- Education on side effect management
- Ongoing monitoring and follow-up
- A direct point of contact for questions or concerns
This level of support helps ensure that PT-141 is used safely and effectively as part of a broader sexual wellness strategy.

PT-141 vs Other Female Libido Treatments
Women experiencing low sexual desire are often presented with limited or incomplete treatment options. Many of these approaches focus on hormone levels or physical response rather than the neurological mechanisms that initiate sexual desire. PT-141 represents a fundamentally different category of treatment.
PT-141 vs Hormone Therapy and Bioidentical Hormones
Hormone replacement therapy and bioidentical hormones are commonly prescribed to address symptoms associated with hormonal decline, perimenopause, or menopause. These treatments aim to restore estrogen, progesterone, or testosterone levels to relieve systemic symptoms.
However, hormone therapy does not directly stimulate the brain’s arousal pathways responsible for sexual motivation.
Key differences include:
- Hormonal therapies adjust circulating hormone levels
- PT-141 activates melanocortin receptors in the brain
- Hormone therapy may take weeks to show effects
- PT-141 is used on demand, prior to sexual activity
For some patients, hormone therapy improves energy, mood, or vaginal comfort but does not restore sexual desire. PT-141 may be used when hormonal therapies alone are insufficient or as part of a broader treatment plan.
PT-141 vs Daily Libido Medications
Some medications for female sexual desire disorder require daily use and gradual accumulation in the body. While effective for certain patients, daily dosing can be associated with systemic side effects, medication fatigue, and limited flexibility.
Because PT-141 works through the nervous system rather than hormone modulation, it may be suitable for women who prefer an as-needed option or who have not tolerated daily medications well.
A Complementary, Not Competitive, Approach
PT-141 is not necessarily a replacement for other treatments. In many cases, it complements existing therapies by addressing the neurological component of sexual desire, while other treatments address hormonal, metabolic, or emotional factors.
This flexibility enables clinicians to tailor treatment strategies to individual patient needs, rather than adopting a one-size-fits-all approach.
How Boston Medical Group Uses PT-141 for Women
Boston Medical Group approaches female sexual health through comprehensive evaluation and individualized care. Rather than treating symptoms in isolation, BMG focuses on identifying the factors contributing to sexual desire disorder and building treatment plans that address those factors safely and effectively.
Personalized Evaluation and Treatment Planning
Before PT-141 is prescribed, patients undergo a detailed assessment that may include:
- Review of medical history and current medications
- Evaluation of sexual symptoms and associated distress
- Discussion of prior hormone therapy or treatments
- Screening for metabolic health concerns such as diabetes
This evaluation ensures that PT-141 is appropriate and that other contributing factors are identified and addressed.
PT-141 as Part of a Broader Sexual Wellness Strategy
At Boston Medical Group, PT-141 is offered in the form of Bremetide™ and may be incorporated alongside other therapies rather than used in isolation. Depending on the patient’s needs, treatment plans may also include:
- Hormonal therapies or bioidentical hormones, when indicated
- Lifestyle and metabolic health optimization
- Weight management strategies, including medications such as semaglutide or tirzepatide, when appropriate
- Ongoing education and follow-up through a dedicated support team
This integrated approach allows clinicians to address hormonal decline, nervous system signaling, and overall sexual wellness together.
Is PT-141 Right for You?
PT-141 is not intended for every woman experiencing changes in libido, but it may be an effective option for those whose symptoms align with a diagnosed sexual desire disorder and who have not found relief through other approaches.
You may be a good candidate for PT-141 if you experience:
- Persistent low sexual desire that causes emotional distress
- Diminished interest in sexual activity despite a supportive relationship
- Normal or treated hormone levels with ongoing symptoms
- Limited improvement from hormone therapy or lifestyle changes
- A preference for an on-demand treatment rather than daily medication
PT-141 may be especially helpful for women whose symptoms are linked to nervous system signaling rather than blood flow or estrogen deficiency alone. This includes women affected by stress-related sexual dysfunction, aging-related neurological changes, or genetic predispositions involving receptor sensitivity in the brain.
That said, treatment decisions should always be guided by a qualified medical professional. A thorough evaluation is essential to rule out other medical conditions, ensure safety, and determine whether PT-141 fits into a broader sexual wellness plan.
Start Your PT-141 Consultation with Boston Medical Group
If low sexual desire is affecting your confidence, relationships, or quality of life, you don’t have to navigate it alone. Boston Medical Group provides discreet, medically supervised care for women seeking evidence-based solutions for sexual desire disorders and overall sexual wellness.
Through a personalized consultation, BMG clinicians evaluate your symptoms, medical history, hormone status, and overall health to determine whether PT-141 may be appropriate for you. Treatment plans are tailored to each patient and supported by an experienced medical support team that remains available throughout your care.
Sexual health is an important part of overall well-being. With the right medical guidance and a personalized approach, meaningful improvement is possible.
To learn more about PT-141 for women or to schedule a confidential consultation, contact Boston Medical Group today and take the next step toward restoring sexual confidence and wellness.

Frequently Asked Questions About PT-141 for Women
Does PT-141 work if my hormone levels are normal?
Yes. PT-141 does not work by changing hormone levels. It acts on melanocortin receptors in the brain, making it effective even when estrogen, testosterone, or other hormone levels are within normal ranges.
Can PT-141 be used during perimenopause or menopause?
PT-141 is FDA-approved for premenopausal women only and not postmenopausal women, and clinicians may consider its use in perimenopausal women on a case-by-case basis. Menopausal status, hormone therapy use, and cardiovascular health should all be evaluated before treatment.
How quickly does PT-141 work?
Most patients begin to notice effects within 30 to 60 minutes after injection. Because it targets the central nervous system, the experience often feels like a gradual increase in sexual interest rather than a sudden physical response.
Can PT-141 be combined with other treatments?
Yes. PT-141 may be used alongside hormone replacement therapy, bioidentical hormones, or other medical treatments when appropriate. It can also complement metabolic health strategies, including weight management programs involving medications such as semaglutide or tirzepatide, when clinically indicated.